SUBA® technology delivers approximately double the bioavailability of conventional 100 mg itraconazole capsules in a smaller 65 mg dose1. This breakthrough innovation enhances the solubility and absorption of poorly soluble drugs such as conventional itraconazole1.
(Size of capsules in image do not reflect actual TOLSURA capsules size.)
WHAT IS SUBA® TECHNOLOGY?
THE SCIENCE OF SUBA®
The novel SUBA® process utilizes a unique spray-drying technology which combines the drug and a polymer solution to generate an amorphous solid dispersion powder.
This produces microencapsulated nanoparticles of itraconazole dispersed in a polymer matrix, instead of the conventional crystalline form.
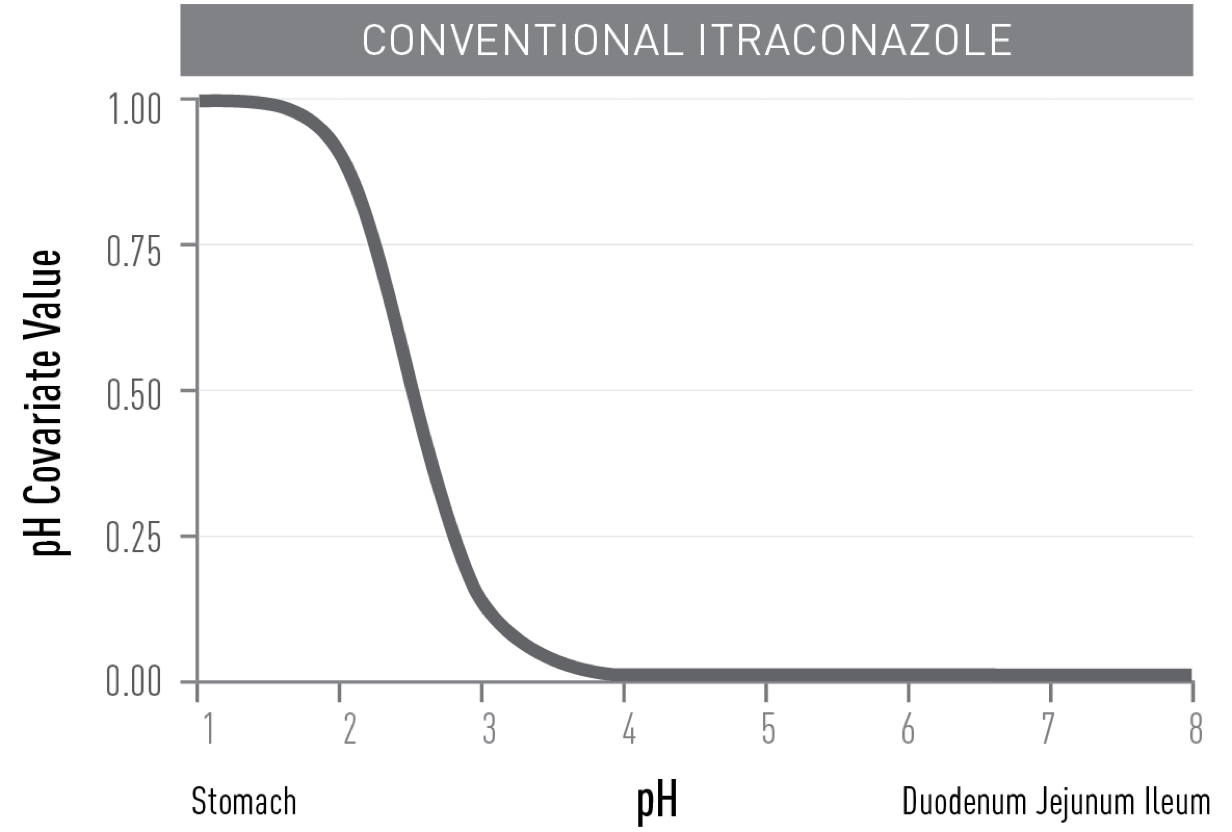
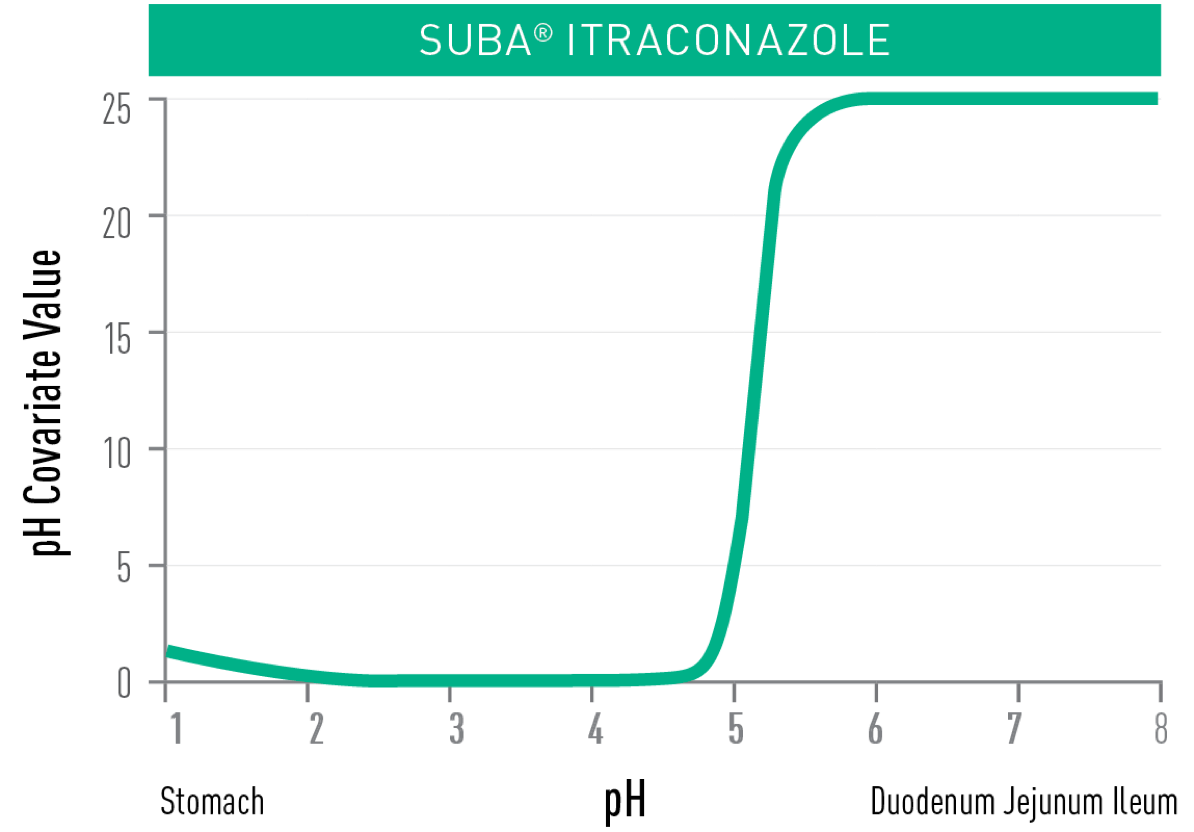
UNLIKE CONVENTIONAL ITRACONAZOLE,
TOLSURA is less soluble in the acidic environment of the stomach and soluble in the higher pH of the small intestine, resulting in improved absorption2.

Crystalline itraconazole


SUBA® itraconazole solid dispersion
TOLSURA TAKES ON CONVENTIONAL ITRACONAZOLE3
INTERIM FINDINGS
Pharmacokinetic results to Day 42 of treatment from a head-to-head clinical trial
TOLSURA SHOWS LESS PATIENT VARIABILITY than conventional itraconazole1 (Figure)
- N=2 patients with high exposure in the TOLSURA group received dose reductions
10% of conventional itraconazole patients had not achieved therapeutic levels of itraconazole by Day 42; 100% of TOLSURA patients were above the therapeutic threshold3
TOLSURA was safe and well tolerated3
- 50% less gastrointestinal events in the TOLSURA group (13%) than the conventional itraconazole group (26%)
- Low rate of abnormal LFTs in both treatment groups
STUDY DESIGN
- Subjects with endemic mycoses (including but not limited to histoplasmosis, blastomycosis) who have had <14 days of prior antifungal therapy
- Life-threatening or CNS disease excluded
- A prospective, multi-center, randomized, open-label parallel arm study
- TOLSURA 130 mg PO BID after 3-day loading dose (130mg PO TID)
- Sporanox, supplied as generic itraconazole capsules (Patriot), 200 mg PO BID after 3-day loading dose (200mg PO TID)
- Clinical Assessments: Days 7, 14, 28, 42, 84, 180
- Pharmacokinetics: Days 7, 14, 42
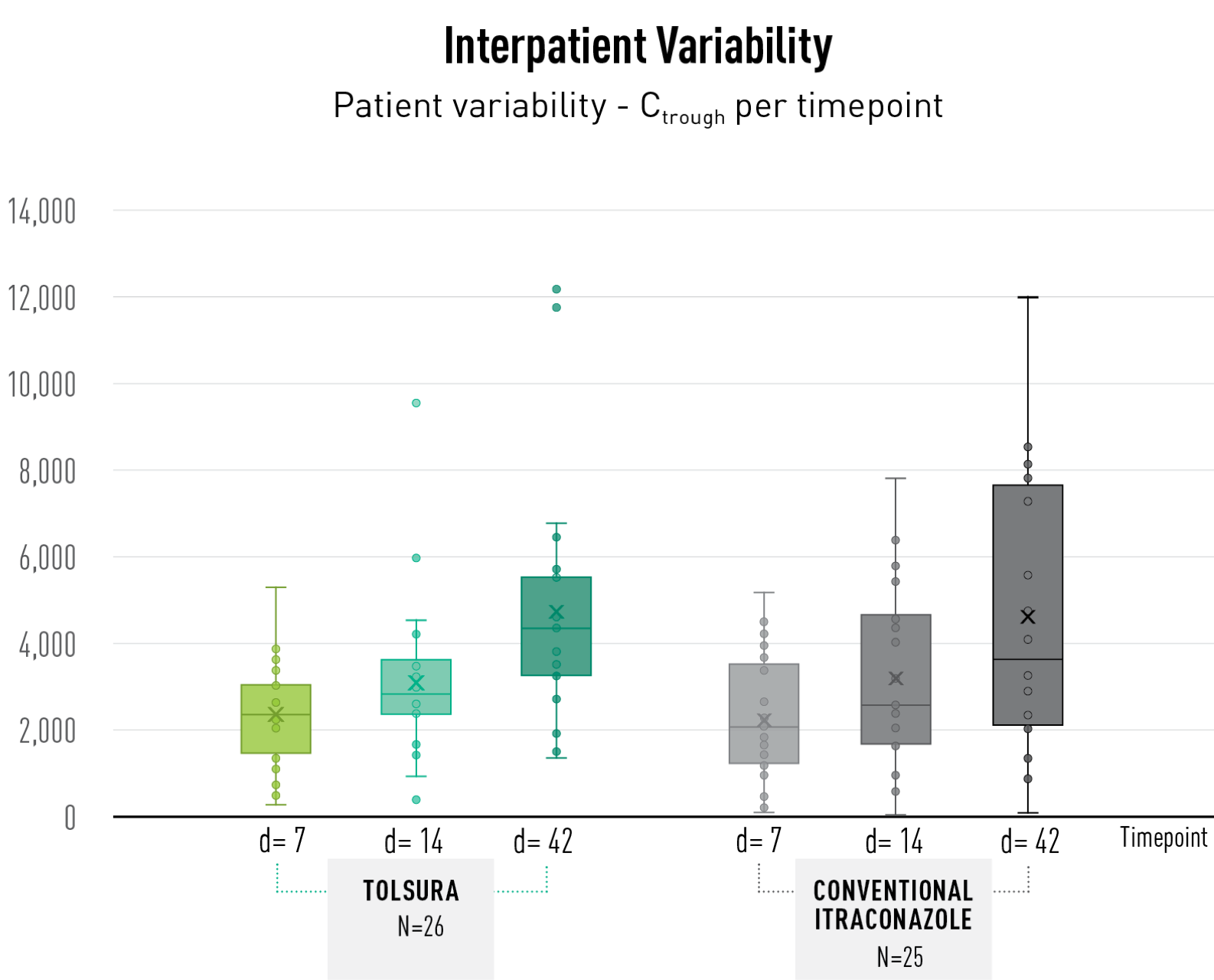
To date, pharmacokinetic data is available through Day 42 of treatment (N=51; histoplasmosis or blastomycosis patients). Blood serum levels of itraconazole and hydroxyitraconazole were measured on Days 7, 14 and 42 and plotted to compare the variability among patients. All patients in both treatment arms were initiated with a loading dose. As expected, dosing adjustments were needed in some patients especially at the start of therapy.
At each timepoint, and by day 42, interpatient variability is less with TOLSURA than with conventional itraconazole.
WHAT DOES THIS MEAN FOR YOUR PATIENTS?
High variability in the absorption of conventional itraconazole formulations can result in varying levels of safety and efficacy if dosing is not managed on an individual basis4.
TOLSURA WITH SUBA® TECHNOLOGY CAN HELP. HERE’S HOW:
- TOLSURA IS UP TO 90% BIOAVAILABLEwith improved overall absorption of conventional itraconazole and consistent plasma levels1
- ACHIEVING THERAPEUTIC LEVELS5,6:of TOLSURA 65 mg subjects*
COMPARED TO JUST
of conventional 100 mg subjects*This difference represents the percentage of subjects in a steady-state PK study above the geometric mean of the conventional itraconazole plasma trough level (1,034 ng/mL)
- WITH TOLSURA THERAPEUTIC LEVELS ARE ACHIEVED AND MAINTAINED in both fed and
fasted conditions7.
(FDA dosing instructions require taking TOLSURA with food)
- THE ABSORPTION OF TOLSURA IS NOT REDUCED† with proton pump inhibitors as the
drug is released in the small intestine5,7,8.
†Monitor for side effects as dosing with a proton pump inhibitor may increase peak and overall exposure of itraconazole
- TOLSURA DELIVERS CONSISTENT BLOOD LEVELS OF ITRACONAZOLE for lower patient variability2,5.

TOLSURA — RIGHT ON TARGET
Learn about TOLSURA’s efficacy

Indications and Important
Safety Information
BOXED WARNING
WARNING: CONGESTIVE HEART FAILURE and DRUG INTERACTIONS
- » CONGESTIVE HEART FAILURE
TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur or worsen during administration of TOLSURA, reassess the benefit and risk of continuing treatment.
- » DRUG INTERACTIONS
- Co-administration of certain drugs that are metabolized by human CYP3A4 enzymes are contraindicated with TOLSURA because plasma concentrations of such drugs are increased, which may also increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs.
- Co-administration with colchicine, fesoterodine and solifenacin is contraindicated in subjects with varying degrees of renal or hepatic impairment, and
- Co-administration with eligustat is contraindicated in subjects taking strong or moderate CYP2D6 inhibitors.
- Increased plasma concentrations of some of these drugs caused by co-administration with TOLSURA can lead to QT prolongation and/or ventricular tachyarrhythmias, including occurrences of torsades de pointes, a potentially fatal arrhythmia.
Indications and Usage
TOLSURA is an azole antifungal indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised adult patients:
- Blastomycosis, pulmonary and extrapulmonary
- Histoplasmosis, including chronic cavitary pulmonary disease and disseminated, non-meningeal histoplasmosis, and
- Aspergillosis, pulmonary and extrapulmonary, in patients who are intolerant of or who are refractory to amphotericin B therapy
Limitations of Use:
TOLSURA is not indicated for the treatment of onychomycosis.
TOLSURA is NOT interchangeable or substitutable with other itraconazole products.
Contraindications
Co-administration with certain drugs that either affect metabolism of itraconazole or whose metabolism is affected by itraconazole Hypersensitivity to itraconazole
Warnings and Precautions
- Hepatotoxicity: Serious hepatotoxicity, including liver failure and death, were reported with the use of itraconazole. Discontinue treatment if signs of liver dysfunction occur
- Cardiac Dysrhythmias: Life-threatening cardiac dysrhythmias and/or sudden death have occurred in patients using certain drugs that are metabolized by human CYP450 enzymes concomitantly with oral itraconazole and/or other CYP3A4 inhibitors.
- Peripheral Neuropathy: This has been reported in patients on long-term therapy with itraconazole. Monitor and promptly evaluate neurologic symptoms
- Hearing loss: Reversible or permanent hearing loss has been reported in patients. Discontinue treatment if hearing loss occurs
Adverse Reactions
Most common adverse reactions (incidence ≥ 1%) are nausea, rash, vomiting, edema, headache, diarrhea, fatigue, fever, pruritus, hypertension, abnormal hepatic function, abdominal pain, dizziness, hypokalemia, anorexia, malaise, decreased libido, somnolence, albuminuria, and impotence.
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For more information, please see full Prescribing Information and Patient Information Leaflet.
REFERENCES:
- Data on File. Mayne Pharma, Inc. 2017.
- Abuhelwa AY, Foster DJ, Mudge S, Hayes D, Upton RN. Population pharmacokinetic modeling of itraconazole and hydroxyitraconazole for oral SUBA-itraconazole and sporanox capsule formulations in healthy subjects in fed and fasted states. Antimicrob Agents Chemother. 2015;59(9):5681-5696. doi:10.1128/AAC.00973-15.
- Pappas PG, et al. MSG-15 Pharmacokinetic, Adverse Events and tolerability data from an open label randomized clinical trial comparing oral SUBA-itraconazole to conventional itraconazole for treatment of endemic mycosis. IDWeek 2020, Abstract O-144. ClinicalTrials.gov Identifier: NCT03572049.
- Prentice AG, Glasmacher A. J Antimicrob Chemother. 2005;56(Suppl 1):i17-i22.
- TOLSURA® Product Information. Mayne Pharma. Greenville, NC. 12/2018.
- Mudge S, Burnett BP. Pharmacokinetic Analysis of SUBA™-itraconazole Capsules Compared to Conventional Itraconazole Capsules for a 3-Day Loading Dose Regimen and After 15 Days of Administration. Poster presented (#L0043) at 29th European Congress of Clinical Mi¬crobiology & Infectious Diseases, Amsterdam, Netherlands. April 13-16, 2019.
- Lindsay J, et al. Antimicrob Agents Chemother. 2018;62(12)e01723-18.
- Data on File. Clinical study report MPG 017.
Indications and Important
Safety Information
BOXED WARNING
WARNING: CONGESTIVE HEART FAILURE and DRUG INTERACTIONS
- » CONGESTIVE HEART FAILURE
TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur or worsen during administration of TOLSURA, reassess the benefit and risk of continuing treatment.
- » DRUG INTERACTIONS
- Co-administration of certain drugs that are metabolized by human CYP3A4 enzymes are contraindicated with TOLSURA because plasma concentrations of such drugs are increased, which may also increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs.
- Co-administration with colchicine, fesoterodine and solifenacin is contraindicated in subjects with varying degrees of renal or hepatic impairment, and
- Co-administration with eligustat is contraindicated in subjects taking strong or moderate CYP2D6 inhibitors.
- Increased plasma concentrations of some of these drugs caused by co-administration with TOLSURA can lead to QT prolongation and/or ventricular tachyarrhythmias, including occurrences of torsades de pointes, a potentially fatal arrhythmia.
Indications and Usage
TOLSURA is an azole antifungal indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised adult patients:
- Blastomycosis, pulmonary and extrapulmonary
- Histoplasmosis, including chronic cavitary pulmonary disease and disseminated, non-meningeal histoplasmosis, and
- Aspergillosis, pulmonary and extrapulmonary, in patients who are intolerant of or who are refractory to amphotericin B therapy
Limitations of Use:
TOLSURA is not indicated for the treatment of onychomycosis.
TOLSURA is NOT interchangeable or substitutable with other itraconazole products.
Contraindications
Co-administration with certain drugs that either affect metabolism of itraconazole or whose metabolism is affected by itraconazole Hypersensitivity to itraconazole
Warnings and Precautions
- Hepatotoxicity: Serious hepatotoxicity, including liver failure and death, were reported with the use of itraconazole. Discontinue treatment if signs of liver dysfunction occur
- Cardiac Dysrhythmias: Life-threatening cardiac dysrhythmias and/or sudden death have occurred in patients using certain drugs that are metabolized by human CYP450 enzymes concomitantly with oral itraconazole and/or other CYP3A4 inhibitors.
- Peripheral Neuropathy: This has been reported in patients on long-term therapy with itraconazole. Monitor and promptly evaluate neurologic symptoms
- Hearing loss: Reversible or permanent hearing loss has been reported in patients. Discontinue treatment if hearing loss occurs
Adverse Reactions
Most common adverse reactions (incidence ≥ 1%) are nausea, rash, vomiting, edema, headache, diarrhea, fatigue, fever, pruritus, hypertension, abnormal hepatic function, abdominal pain, dizziness, hypokalemia, anorexia, malaise, decreased libido, somnolence, albuminuria, and impotence.
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For more information, please see full Prescribing Information and Patient Information Leaflet.
