ADVANCED ANTIFUNGAL INNOVATION YOU CAN DEPEND ON
FOR ENDEMIC AND OPPORTUNISTIC FUNGAL INFECTIONS TOLSURA 65 mg capsules offer the efficacy of itraconazole capsules with improved absorption and less variability in plasma levels 1
RIGHT ON TARGET

When Ctrough was corrected for dose administration, the relative bioavailability of TOLSURA was nearly 2x that of conventional itraconazole capsules.
| Parameter* | TOLSURA 130 mg twice daily (2 x 65 mg Capsules) | Itraconazole 200 mg twice daily (2 x 100 mg Capsules) |
|---|---|---|
| AUCO-tau (hr•ng/ml) | 15,600 ± 3,700 | 14,900 ± 3,800 |
| Cthrough (ng/ml) | 1,200 ± 400 | 1,000 ± 300 |
| Cmax (ng/ml) | 1,600 ± 400 | 1,500 ± 400 |
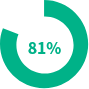
Patients with a mean Ctrough level of >1,034 ng/mL6,†
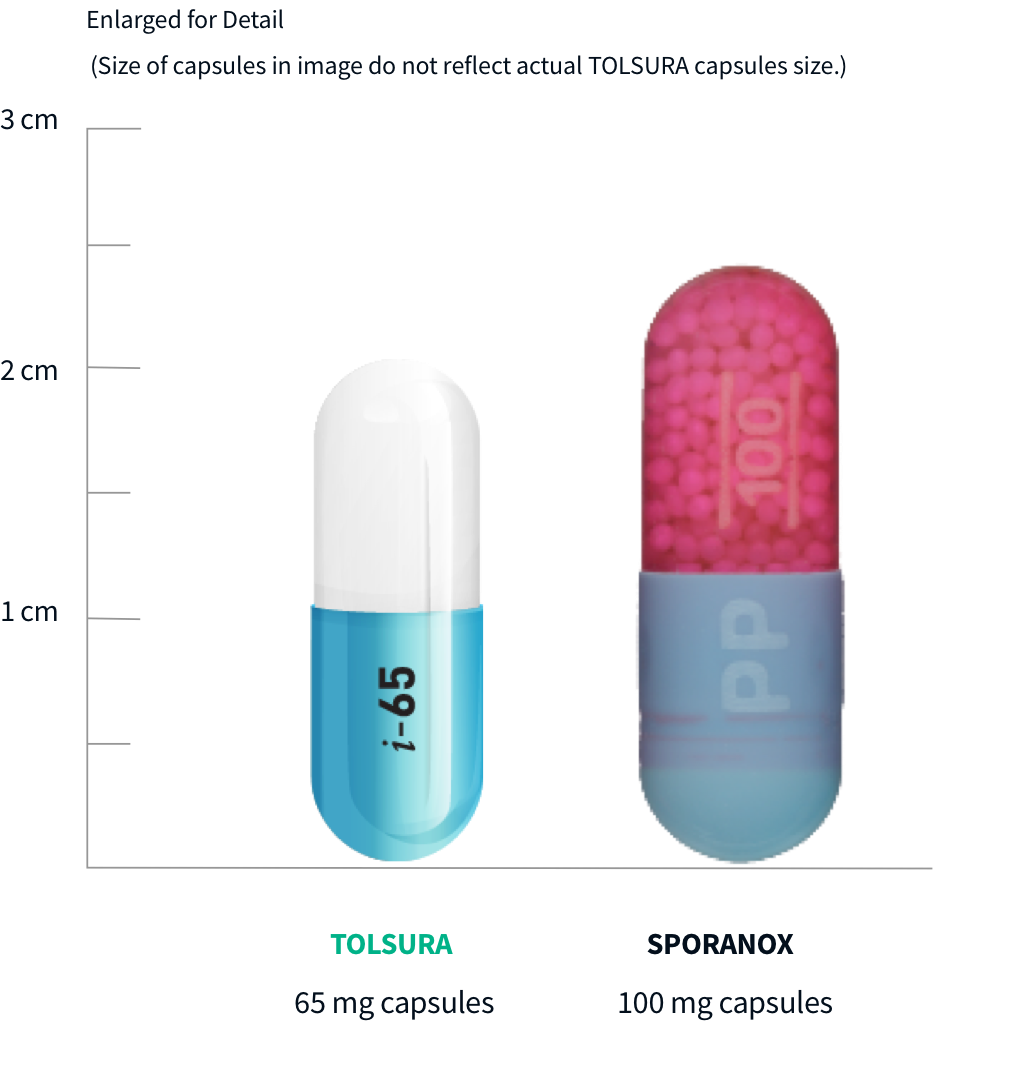
 TOLSURA 65 mg‡
TOLSURA 65 mg‡ Itraconzole 100 mg1,6
Itraconzole 100 mg1,6The bioavailability of TOLSURA at steady state, under fed conditions was determined by a comparative pharmacokinetic study with 100 mg itraconazole capsules. 16 healthy subjects were dosed orally immediately after a meal, twice-daily for 14.5 days.
FOR ENDEMIC AND OPPORTUNISTIC FUNGAL INFECTIONS TOLSURA 65 mg capsules offer the efficacy of itraconazole capsules with improved absorption and less variability in plasma levels 1

approximately twice that of conventional itraconazole, with improved absorption and reduced plasma variability2.

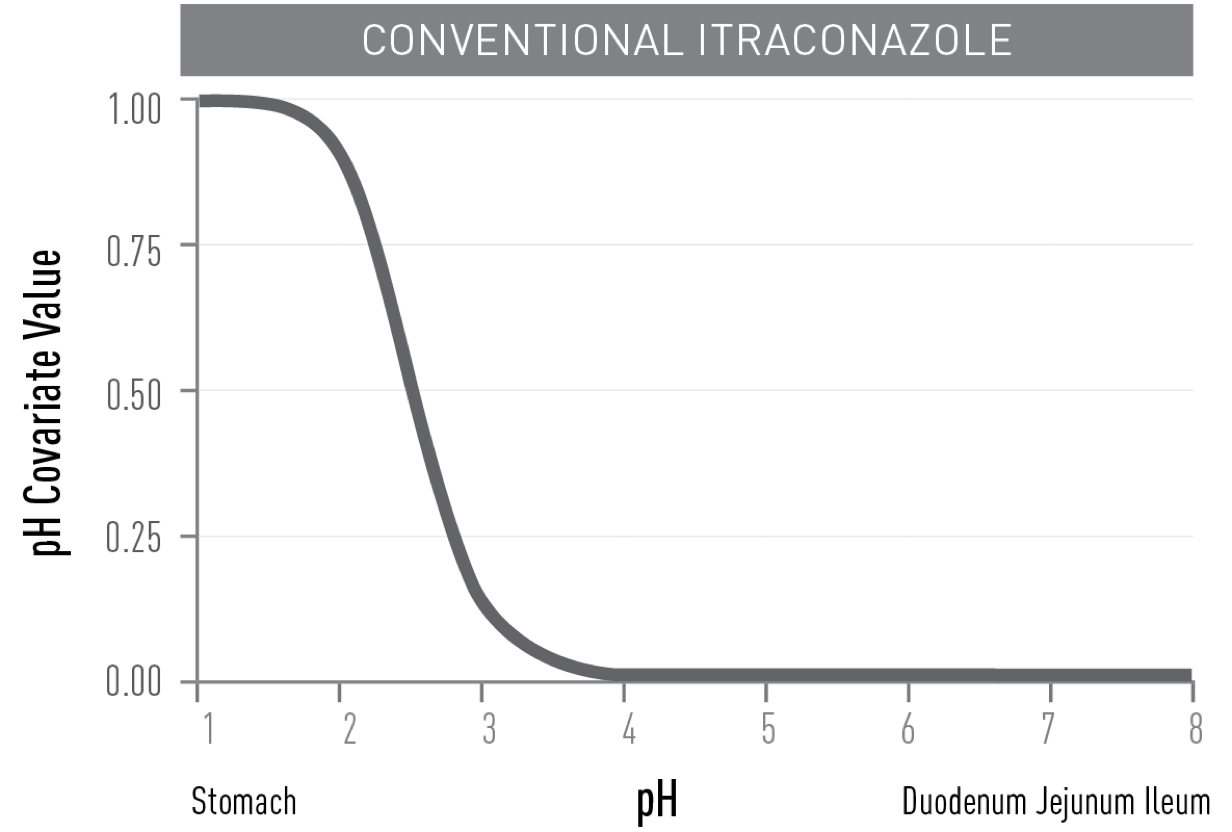
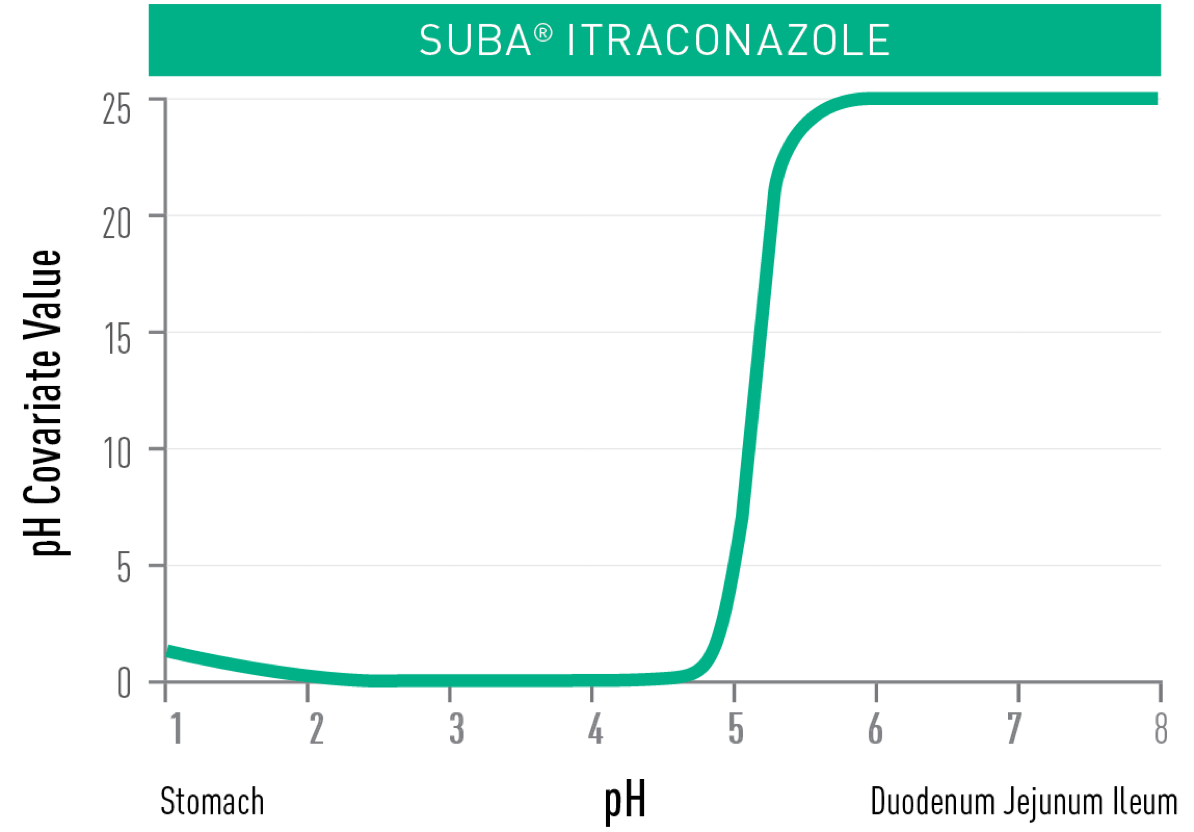
in the higher pH of the upper small intestine, resulting in improved absorption3 which is not reduced with proton pump inhibitor use1,4,5,*.

compared to 44% taking conventional itraconazole capsules.1,6 With TOLSURA, therapeutic levels are achieved and maintained in both fed and fasted states (FDA dosing instructions require taking with food)4,6,7.
Indications and Important
Safety Information
BOXED WARNING
WARNING: CONGESTIVE HEART FAILURE and DRUG INTERACTIONS
TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur or worsen during administration of TOLSURA, reassess the benefit and risk of continuing treatment.
Indications and Usage
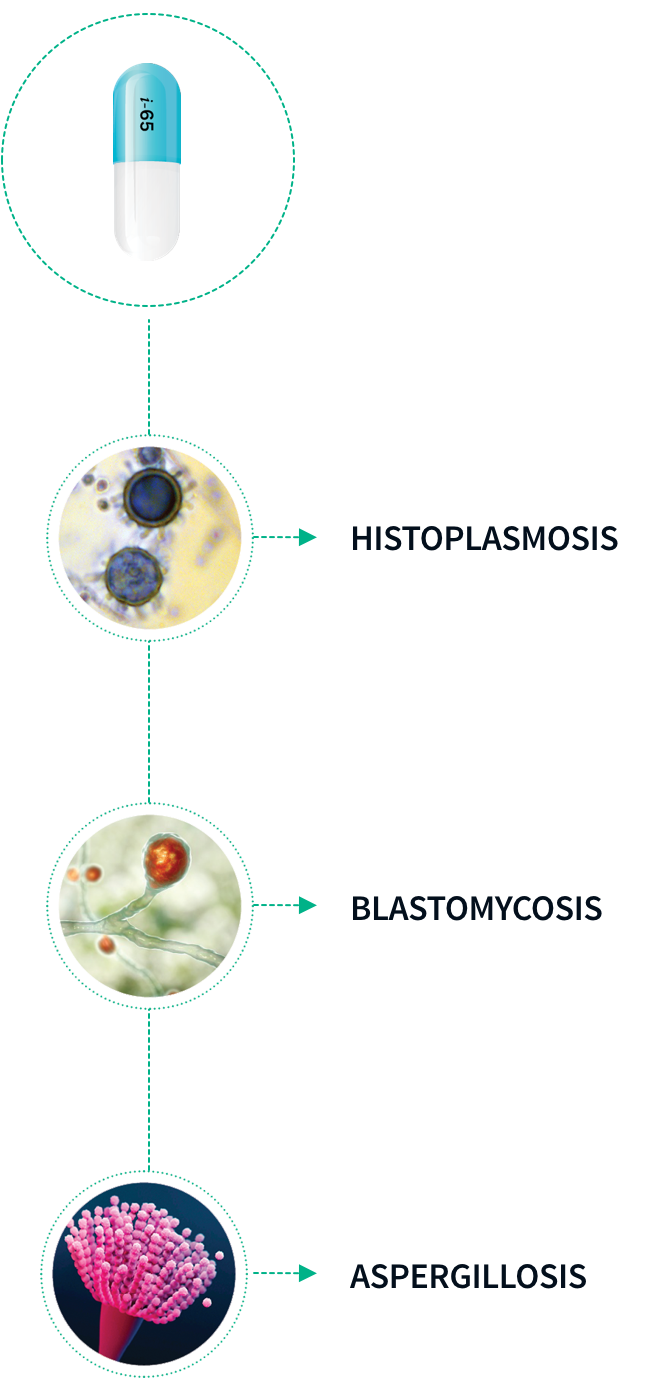
TOLSURA is an azole antifungal indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised adult patients:
Limitations of Use:
TOLSURA is not indicated for the treatment of onychomycosis.
TOLSURA is NOT interchangeable or substitutable with other itraconazole products.
Contraindications
Co-administration with certain drugs that either affect metabolism of itraconazole or whose metabolism is affected by itraconazole Hypersensitivity to itraconazole
Warnings and Precautions
Adverse Reactions
Most common adverse reactions (incidence ≥ 1%) are nausea, rash, vomiting, edema, headache, diarrhea, fatigue, fever, pruritus, hypertension, abnormal hepatic function, abdominal pain, dizziness, hypokalemia, anorexia, malaise, decreased libido, somnolence, albuminuria, and impotence.
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For more information, please see full Prescribing Information and Patient Information Leaflet.
Indications and Important
Safety Information
BOXED WARNING
WARNING: CONGESTIVE HEART FAILURE and DRUG INTERACTIONS
TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur or worsen during administration of TOLSURA, reassess the benefit and risk of continuing treatment.
Indications and Usage
TOLSURA is an azole antifungal indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised adult patients:
Limitations of Use:
TOLSURA is not indicated for the treatment of onychomycosis.
TOLSURA is NOT interchangeable or substitutable with other itraconazole products.
Contraindications
Co-administration with certain drugs that either affect metabolism of itraconazole or whose metabolism is affected by itraconazole Hypersensitivity to itraconazole
Warnings and Precautions
Adverse Reactions
Most common adverse reactions (incidence ≥ 1%) are nausea, rash, vomiting, edema, headache, diarrhea, fatigue, fever, pruritus, hypertension, abnormal hepatic function, abdominal pain, dizziness, hypokalemia, anorexia, malaise, decreased libido, somnolence, albuminuria, and impotence.
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For more information, please see full Prescribing Information and Patient Information Leaflet.