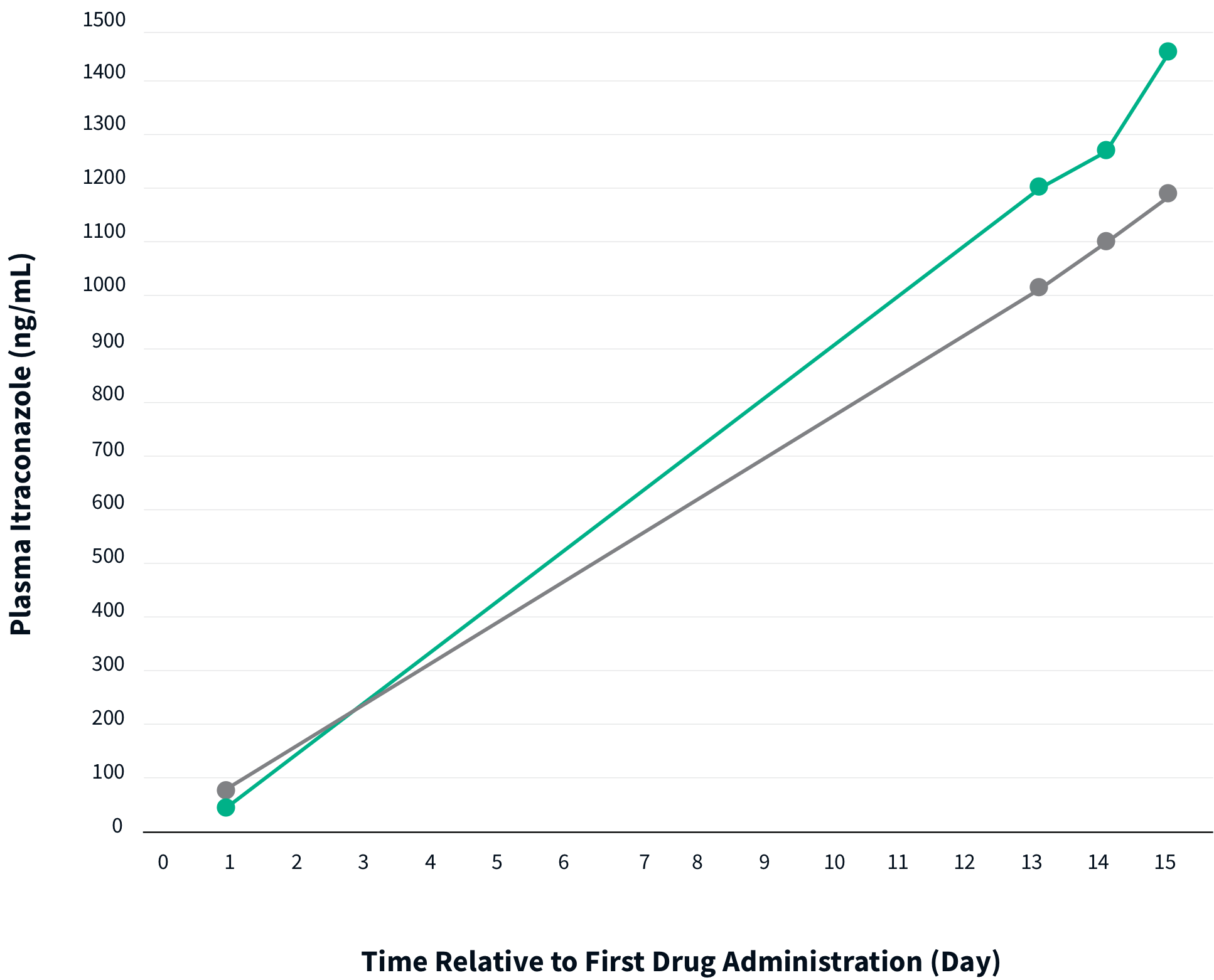
TOLSURA 65 MG DEMONSTRATES ~2X BIOAVAILABILITY VS. SPORANOX® 100 MG1,2

When the Ctrough was corrected for dose administration, the relative bioavailability of TOLSURA was nearly 2X that of Sporanox3.

The relative Ctrough of TOLSURA is greater than SPORANOX with 35 mg less drug per capsule dose2.


TOLSURA
2x 65 mg capsules
n= 16


Sporanox®
2x 100 mg capsules
n= 16
(Size of capsules in image do not reflect actual TOLSURA capsules size.)
TOLSURA 65 MG CAPSULES ARE BIOEQUIVALENT TO SPORANOX® 100 MG CAPSULES2
The bioavailability of TOLSURA 65 mg capsules at steady state, under fed conditions, was determined by a comparative pharmacokinetic study with SPORANOX 100 mg capsules.
TREATMENT A
TOLSURA 65 mg capsules
(2 capsules on Days 1-14 BID, Day 15 QD at steady state)
TREATMENT B
SPORANOX 100 mg capsules
(2 capsules on Days 1-14 BID, Day 15 QD at steady state)
BASED ON STATISTICAL DATASET
| Paparameter | Treatment | n | Arithmetic Mean (CV%) | Geometric Mean | Contrast | Ratio | 90% Confidence Level | Intr-Sbj CV (%) |
|---|---|---|---|---|---|---|---|---|
| Cmax_ss (ng/mL) | A | 16 | 1,667.5 (22) | 1,632.2 | A vs. B | 111.99 | 104.87 - 119.59 | 11 |
| B | 16 | 1,505.1 (27) | 1,457.5 | |||||
| Ctrough (ng/mL) | A | 16 | 1,229.8 (29) | 1,187.4 | A vs. B | 118.16 | 110.20 - 126.69 | 11 |
| B | 16 | 1,034.4 (25) | 1,004.9 | |||||
| AUCtau (hr•ng/mL) | A | 16 | 15,943.6 (23) | 15,562.1 | A vs. B | 110.64 | 104.01 - 117.70 | 10 |
| B | 16 | 14,529.5 (26) | 14,065.1 | |||||
| n | Median | Range | ||||||
| Tmax,ss (hr) | A | 16 | 7.00 | 0.00 - 10.00 | ||||
| B | 16 | 5.00 | 0.00 - 8.00 |

LEARN ABOUT TOLSURA’S IMPROVED ABSORPTION
TOLSURA’s SUBA® technology offers significantly improved solubility in the higher pH of the upper small intestine, resulting in improved absorption1 which is not reduced with proton pump inhibitor use4,5,6,*.
Indications and Important
Safety Information
BOXED WARNING
WARNING: CONGESTIVE HEART FAILURE and DRUG INTERACTIONS
- » CONGESTIVE HEART FAILURE
TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur or worsen during administration of TOLSURA, reassess the benefit and risk of continuing treatment.
- » DRUG INTERACTIONS
- Co-administration of certain drugs that are metabolized by human CYP3A4 enzymes are contraindicated with TOLSURA because plasma concentrations of such drugs are increased, which may also increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs.
- Co-administration with colchicine, fesoterodine and solifenacin is contraindicated in subjects with varying degrees of renal or hepatic impairment, and
- Co-administration with eligustat is contraindicated in subjects taking strong or moderate CYP2D6 inhibitors.
- Increased plasma concentrations of some of these drugs caused by co-administration with TOLSURA can lead to QT prolongation and/or ventricular tachyarrhythmias, including occurrences of torsades de pointes, a potentially fatal arrhythmia.
Indications and Usage
TOLSURA is an azole antifungal indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised adult patients:
- Blastomycosis, pulmonary and extrapulmonary
- Histoplasmosis, including chronic cavitary pulmonary disease and disseminated, non-meningeal histoplasmosis, and
- Aspergillosis, pulmonary and extrapulmonary, in patients who are intolerant of or who are refractory to amphotericin B therapy
Limitations of Use:
TOLSURA is not indicated for the treatment of onychomycosis.
TOLSURA is NOT interchangeable or substitutable with other itraconazole products.
Contraindications
Co-administration with certain drugs that either affect metabolism of itraconazole or whose metabolism is affected by itraconazole Hypersensitivity to itraconazole
Warnings and Precautions
- Hepatotoxicity: Serious hepatotoxicity, including liver failure and death, were reported with the use of itraconazole. Discontinue treatment if signs of liver dysfunction occur
- Cardiac Dysrhythmias: Life-threatening cardiac dysrhythmias and/or sudden death have occurred in patients using certain drugs that are metabolized by human CYP450 enzymes concomitantly with oral itraconazole and/or other CYP3A4 inhibitors.
- Peripheral Neuropathy: This has been reported in patients on long-term therapy with itraconazole. Monitor and promptly evaluate neurologic symptoms
- Hearing loss: Reversible or permanent hearing loss has been reported in patients. Discontinue treatment if hearing loss occurs
Adverse Reactions
Most common adverse reactions (incidence ≥ 1%) are nausea, rash, vomiting, edema, headache, diarrhea, fatigue, fever, pruritus, hypertension, abnormal hepatic function, abdominal pain, dizziness, hypokalemia, anorexia, malaise, decreased libido, somnolence, albuminuria, and impotence.
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For more information, please see full Prescribing Information and Patient Information Leaflet.
References:
- Data on File. Mayne Pharma, Inc. 2017.
- Data on file. Mayne Pharma, Inc. Clinical study report MPG015.
- hompson GR, et al. Antimicrob Agents Chemother. 2020;64(8):e00400-20.
- TOLSURA® Product Information. Mayne Pharma. Greenville, NC. 12/2018.
- Lindsay, J., Mudge, S., & Thompson III, G. R. (2018). Effects of food and omeprazole on a novel formulation of super bioavailability itraconazole in healthy subjects. Antimicrobial Agents and Chemotherapy, 62(12).
- Data on file. Mayne Pharma, Inc. Clinical study report MPG016.
Indications and Important
Safety Information
BOXED WARNING
WARNING: CONGESTIVE HEART FAILURE and DRUG INTERACTIONS
- » CONGESTIVE HEART FAILURE
TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur or worsen during administration of TOLSURA, reassess the benefit and risk of continuing treatment.
- » DRUG INTERACTIONS
- Co-administration of certain drugs that are metabolized by human CYP3A4 enzymes are contraindicated with TOLSURA because plasma concentrations of such drugs are increased, which may also increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs.
- Co-administration with colchicine, fesoterodine and solifenacin is contraindicated in subjects with varying degrees of renal or hepatic impairment, and
- Co-administration with eligustat is contraindicated in subjects taking strong or moderate CYP2D6 inhibitors.
- Increased plasma concentrations of some of these drugs caused by co-administration with TOLSURA can lead to QT prolongation and/or ventricular tachyarrhythmias, including occurrences of torsades de pointes, a potentially fatal arrhythmia.
Indications and Usage
TOLSURA is an azole antifungal indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised adult patients:
- Blastomycosis, pulmonary and extrapulmonary
- Histoplasmosis, including chronic cavitary pulmonary disease and disseminated, non-meningeal histoplasmosis, and
- Aspergillosis, pulmonary and extrapulmonary, in patients who are intolerant of or who are refractory to amphotericin B therapy
Limitations of Use:
TOLSURA is not indicated for the treatment of onychomycosis.
TOLSURA is NOT interchangeable or substitutable with other itraconazole products.
Contraindications
Co-administration with certain drugs that either affect metabolism of itraconazole or whose metabolism is affected by itraconazole Hypersensitivity to itraconazole
Warnings and Precautions
- Hepatotoxicity: Serious hepatotoxicity, including liver failure and death, were reported with the use of itraconazole. Discontinue treatment if signs of liver dysfunction occur
- Cardiac Dysrhythmias: Life-threatening cardiac dysrhythmias and/or sudden death have occurred in patients using certain drugs that are metabolized by human CYP450 enzymes concomitantly with oral itraconazole and/or other CYP3A4 inhibitors.
- Peripheral Neuropathy: This has been reported in patients on long-term therapy with itraconazole. Monitor and promptly evaluate neurologic symptoms
- Hearing loss: Reversible or permanent hearing loss has been reported in patients. Discontinue treatment if hearing loss occurs
Adverse Reactions
Most common adverse reactions (incidence ≥ 1%) are nausea, rash, vomiting, edema, headache, diarrhea, fatigue, fever, pruritus, hypertension, abnormal hepatic function, abdominal pain, dizziness, hypokalemia, anorexia, malaise, decreased libido, somnolence, albuminuria, and impotence.
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For more information, please see full Prescribing Information and Patient Information Leaflet.
