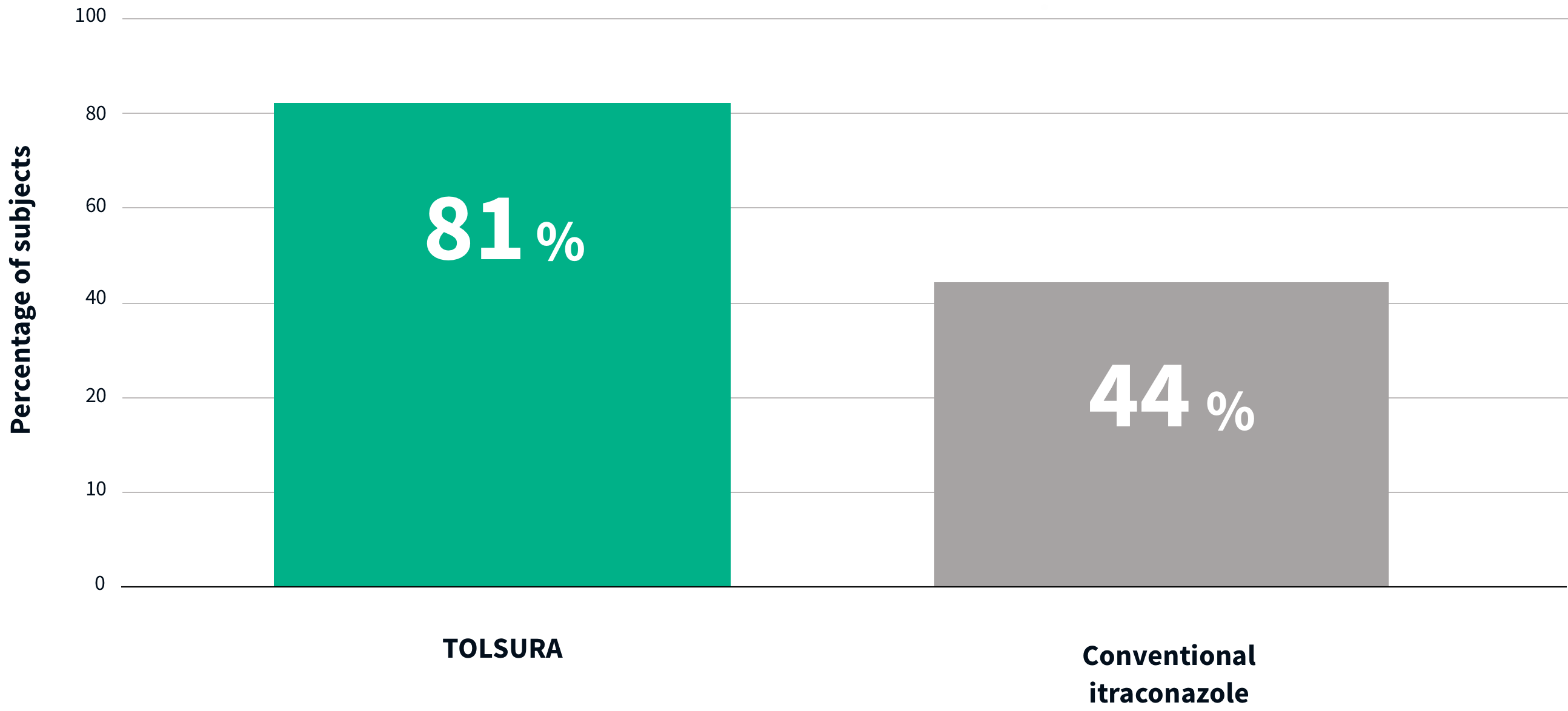
THE MAJORITY OF TOLSURA 65 MG SUBJECTS ACHIEVED THERAPEUTIC LEVELS VS. CONVENTIONAL ITRACONAZOLE PATIENTS1
84% more TOLSURA subjects achieved therapeutic levels vs. subjects on SPORANOX® 100 mg (81% vs 44%)1
TOTAL AND PEAK ITRACONAZOLE EXPOSURE was similar between TOLSURA (65 mg) and SPORANOX (100 mg) treatments1
~2X GREATER RELATIVE BIOAVAILABILITY vs. conventional itraconazole when Ctrough was corrected for dose administration in a fed state2
STEADY STATE CONCENTRATIONS reached within 15 days3
TOLSURA CAN REDUCE PATIENT VARIABILITY AND PRODUCE POTENTIALLY PREDICTABLE RESULTS1
PERCENTAGE OF SUBJECTS WHO REACHED Ctrough LEVEL AT 1,034 ng/ml AT STEADY-STATE
In systemic therapy, itraconazole serum trough levels below 1,000 ng/ml are predictive of therapeutic failure
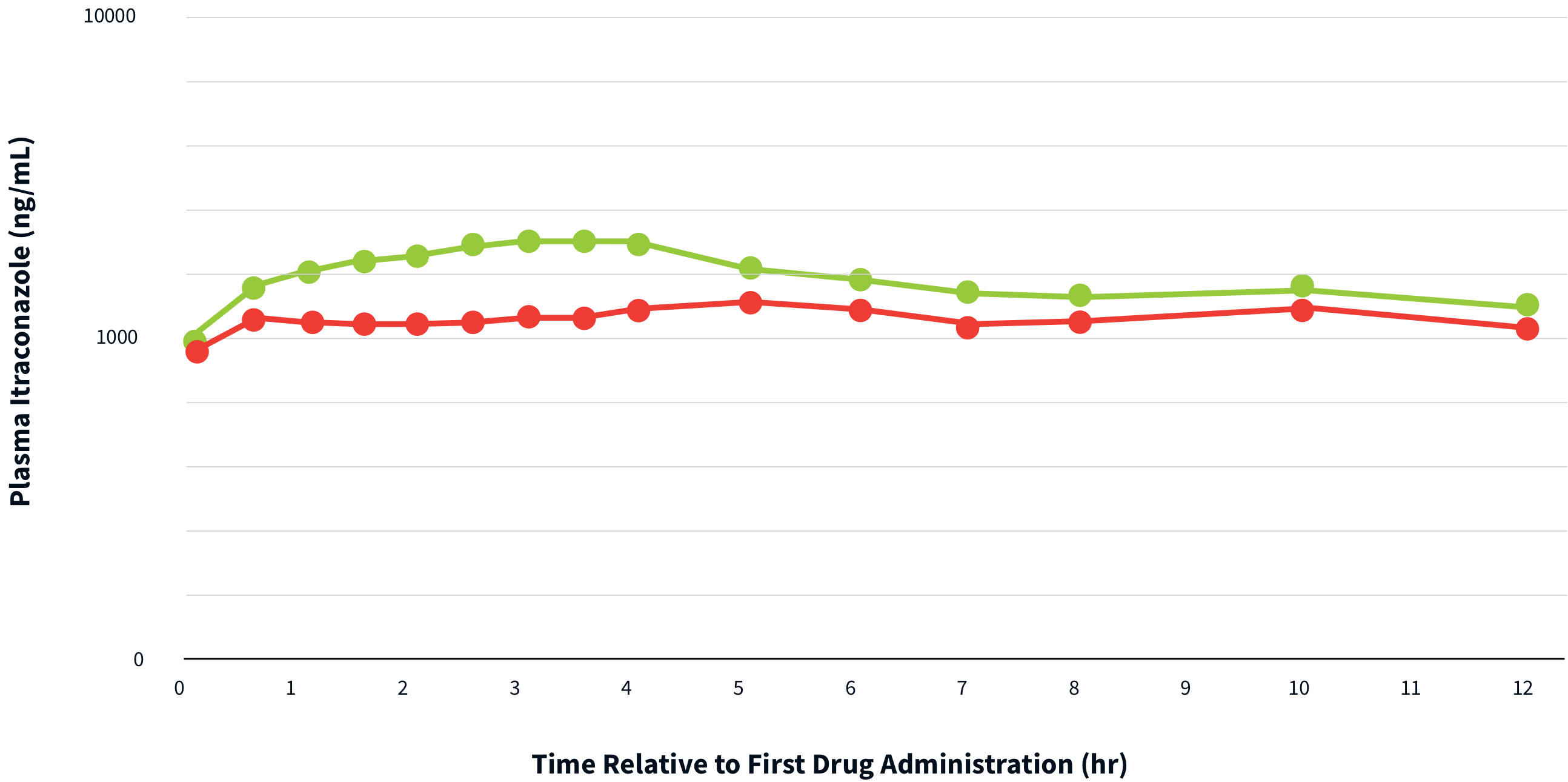
THERAPEUTIC LEVELS ARE ACHIEVED AND MAINTAINED WITH TOLSURA 65 MG CAPSULES IN BOTH FED AND FASTED CONDITIONS4

Peak plasma concentrations of itraconazole after a single dose of TOLSURA are reached after 2 – 6 hours in both fasted and fed states3.

Bioavailability of conventional itraconazole capsules is reduced by 40% when taken without food5††capsule formulation

Serum concentrations below 1,000 ng/ml are predictive of therapeutic failure4.
THE FDA LABEL REQUIRES ADMINISTRATION WITH FOOD.
Conventional itraconazole capsules must be taken with a full meal and, in certain cases, a can of a non-diet cola
FED
TOLSURA
2x 65 mg capsules
FASTED
TOLSURA
2x 65 mg capsules

THE SCIENCE OF SUBA®
 WATCH MECHANISM OF ACTION VIDEO
WATCH MECHANISM OF ACTION VIDEO Indications and Important
Safety Information
BOXED WARNING
WARNING: CONGESTIVE HEART FAILURE and DRUG INTERACTIONS
- » CONGESTIVE HEART FAILURE
TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur or worsen during administration of TOLSURA, reassess the benefit and risk of continuing treatment.
- » DRUG INTERACTIONS
- Co-administration of certain drugs that are metabolized by human CYP3A4 enzymes are contraindicated with TOLSURA because plasma concentrations of such drugs are increased, which may also increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs.
- Co-administration with colchicine, fesoterodine and solifenacin is contraindicated in subjects with varying degrees of renal or hepatic impairment, and
- Co-administration with eligustat is contraindicated in subjects taking strong or moderate CYP2D6 inhibitors.
- Increased plasma concentrations of some of these drugs caused by co-administration with TOLSURA can lead to QT prolongation and/or ventricular tachyarrhythmias, including occurrences of torsades de pointes, a potentially fatal arrhythmia.
Indications and Usage
TOLSURA is an azole antifungal indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised adult patients:
- Blastomycosis, pulmonary and extrapulmonary
- Histoplasmosis, including chronic cavitary pulmonary disease and disseminated, non-meningeal histoplasmosis, and
- Aspergillosis, pulmonary and extrapulmonary, in patients who are intolerant of or who are refractory to amphotericin B therapy
Limitations of Use:
TOLSURA is not indicated for the treatment of onychomycosis.
TOLSURA is NOT interchangeable or substitutable with other itraconazole products.
Contraindications
Co-administration with certain drugs that either affect metabolism of itraconazole or whose metabolism is affected by itraconazole Hypersensitivity to itraconazole
Warnings and Precautions
- Hepatotoxicity: Serious hepatotoxicity, including liver failure and death, were reported with the use of itraconazole. Discontinue treatment if signs of liver dysfunction occur
- Cardiac Dysrhythmias: Life-threatening cardiac dysrhythmias and/or sudden death have occurred in patients using certain drugs that are metabolized by human CYP450 enzymes concomitantly with oral itraconazole and/or other CYP3A4 inhibitors.
- Peripheral Neuropathy: This has been reported in patients on long-term therapy with itraconazole. Monitor and promptly evaluate neurologic symptoms
- Hearing loss: Reversible or permanent hearing loss has been reported in patients. Discontinue treatment if hearing loss occurs
Adverse Reactions
Most common adverse reactions (incidence ≥ 1%) are nausea, rash, vomiting, edema, headache, diarrhea, fatigue, fever, pruritus, hypertension, abnormal hepatic function, abdominal pain, dizziness, hypokalemia, anorexia, malaise, decreased libido, somnolence, albuminuria, and impotence.
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For more information, please see full Prescribing Information and Patient Information Leaflet.
REFERENCES:
- Data on File. Mayne Pharma, Inc. 2017.
- Abuhelwa AY, Foster DJ, Mudge S, Hayes D, Upton RN. Population pharmacokinetic modeling of itraconazole and hydroxyitraconazole for oral SUBA-itraconazole and sporanox capsule formulations in healthy subjects in fed and fasted states. Antimicrob Agents Chemother. 2015;59(9):5681-5696. doi:10.1128/AAC.00973-15.
- Pappas PG, et al. MSG-15 Pharmacokinetic, Adverse Events and tolerability data from an open label randomized clinical trial comparing oral SUBA-itraconazole to conventional itraconazole for treatment of endemic mycosis. IDWeek 2020, Abstract O-144. ClinicalTrials.gov Identifier: NCT03572049.
- Prentice AG, Glasmacher A. J Antimicrob Chemother. 2005;56(Suppl 1):i17-i22.
- TOLSURA® Product Information. Mayne Pharma. Greenville, NC. 12/2018.
- Mudge S, Burnett BP. Pharmacokinetic Analysis of SUBA™-itraconazole Capsules Compared to Conventional Itraconazole Capsules for a 3-Day Loading Dose Regimen and After 15 Days of Administration. Poster presented (#L0043) at 29th European Congress of Clinical Mi¬crobiology & Infectious Diseases, Amsterdam, Netherlands. April 13-16, 2019.
- Lindsay J, et al. Antimicrob Agents Chemother. 2018;62(12)e01723-18.
- Data on File. Clinical study report MPG 017.
Indications and Important
Safety Information
BOXED WARNING
WARNING: CONGESTIVE HEART FAILURE and DRUG INTERACTIONS
- » CONGESTIVE HEART FAILURE
TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur or worsen during administration of TOLSURA, reassess the benefit and risk of continuing treatment.
- » DRUG INTERACTIONS
- Co-administration of certain drugs that are metabolized by human CYP3A4 enzymes are contraindicated with TOLSURA because plasma concentrations of such drugs are increased, which may also increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs.
- Co-administration with colchicine, fesoterodine and solifenacin is contraindicated in subjects with varying degrees of renal or hepatic impairment, and
- Co-administration with eligustat is contraindicated in subjects taking strong or moderate CYP2D6 inhibitors.
- Increased plasma concentrations of some of these drugs caused by co-administration with TOLSURA can lead to QT prolongation and/or ventricular tachyarrhythmias, including occurrences of torsades de pointes, a potentially fatal arrhythmia.
Indications and Usage
TOLSURA is an azole antifungal indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised adult patients:
- Blastomycosis, pulmonary and extrapulmonary
- Histoplasmosis, including chronic cavitary pulmonary disease and disseminated, non-meningeal histoplasmosis, and
- Aspergillosis, pulmonary and extrapulmonary, in patients who are intolerant of or who are refractory to amphotericin B therapy
Limitations of Use:
TOLSURA is not indicated for the treatment of onychomycosis.
TOLSURA is NOT interchangeable or substitutable with other itraconazole products.
Contraindications
Co-administration with certain drugs that either affect metabolism of itraconazole or whose metabolism is affected by itraconazole Hypersensitivity to itraconazole
Warnings and Precautions
- Hepatotoxicity: Serious hepatotoxicity, including liver failure and death, were reported with the use of itraconazole. Discontinue treatment if signs of liver dysfunction occur
- Cardiac Dysrhythmias: Life-threatening cardiac dysrhythmias and/or sudden death have occurred in patients using certain drugs that are metabolized by human CYP450 enzymes concomitantly with oral itraconazole and/or other CYP3A4 inhibitors.
- Peripheral Neuropathy: This has been reported in patients on long-term therapy with itraconazole. Monitor and promptly evaluate neurologic symptoms
- Hearing loss: Reversible or permanent hearing loss has been reported in patients. Discontinue treatment if hearing loss occurs
Adverse Reactions
Most common adverse reactions (incidence ≥ 1%) are nausea, rash, vomiting, edema, headache, diarrhea, fatigue, fever, pruritus, hypertension, abnormal hepatic function, abdominal pain, dizziness, hypokalemia, anorexia, malaise, decreased libido, somnolence, albuminuria, and impotence.
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For more information, please see full Prescribing Information and Patient Information Leaflet.
