HISTOPLASMOSIS
AREA ENDEMIC FOR INFECTION:

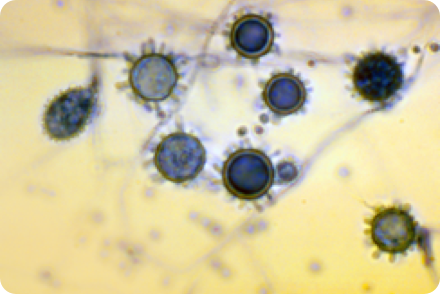
HISTOPLASMA
IDSA RECOMMENDS ITRACONAZOLE FOR HISTOPLASMOSIS1,2
IDSA guidelines describe using antifungals, including itraconazole, for treating cases of histoplasmosis.
HISTOPLASMOSIS
| IDSA GUIDELINES FOR TREATING HISTOPLASMOSIS | |
|---|---|
| Manifestation | Treatment |
| Acute pulmonary histoplasmosis | |
| Mild to moderate | 200 mg itraconazole OD or BID for 6-12 weeks for patients who continue to have symptoms for >1 month |
| Moderately severe to severe | 200 mg itraconazole BID for a total of 12 weeks following 1-2 weeks of Amphotericin B |
| Chronic cavitary pulmonary histoplasmosis | |
| Loading dose of 200 mg itraconazole TID for the first 3 days, followed by 200 mg itraconazole OD or BID for 6-12 weeks for at least 12 months | |
| Pericarditis | |
| Moderately severe to severe | 200 mg itraconazole OD or BID for 6-12 weeks if prednisone used |
| Mediastinal granuloma | |
| Symptomatic | 200 mg itraconazole OD or BID for 6-12 weeks |
| Progressive disseminated histoplasmosis | |
| Mild to moderate | 200 mg itraconazole BID for at least 12 months |
| Moderately severe to severe | 200 mg itraconazole BID for at least 12 months following 1-2 weeks of Amphotericin B |
| CNS histoplasmosis | |
| Mild to moderate | 200 mg itraconazole BID or TID for at least 12 months following 1-2 weeks of Amphotericin B |
WHEN HISTOPLASMOSIS PATIENTS WERE TREATED WITH ORAL ITRACONAZOLE3:
81%
ACHIEVED TREATMENT SUCCESS
86%
ACHIEVED TREATMENT SUCCESS WHEN TREATED AT THERAPEUTIC LEVELS FOR AT LEAST 2 MONTHS
9 MONTHS
MEDIAN DURATION TREATMENT TIME FOR PATIENTS WHO WERE CONSIDERED A SUCCESS
BLASTOMYCOSIS
AREA ENDEMIC FOR INFECTION:

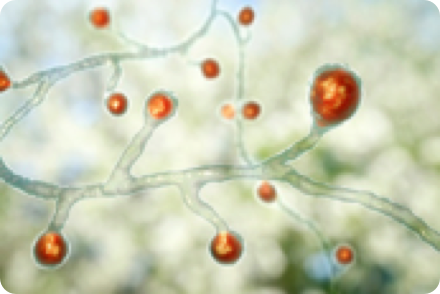
BLASTOMYCOSIS
IDSA RECOMMENDS ITRACONAZOLE FOR BLASTOMYCOSIS1,2
IDSA guidelines describe using antifungals, including itraconazole, for treating cases of blastomycosis.
BLASTOMYCOSIS
| BLASTOMYCOSIS | |
|---|---|
| Manifestation | Treatment |
| Pulmonary blastomycosis | |
| Mild to moderate | 200 mg itraconazole OD or BID for 6-12 months |
| Moderately severe to severe | 200 mg itraconazole BID for a total of 12 weeks following 1-2 weeks of Amphotericin B |
| Disseminated blastomycosis | |
| Mild to moderate | 200 mg itraconazole BID for 6-12 months |
| Moderately severe to severe | 200 mg itraconazole BID for 12 months following 1-2 weeks of Amphotericin B |
| CNS blastomycosis | |
| oral azole therapy for 1 year following 4-6 weeks of Amphotericin B | |
| Immunosuppressed patients | |
| 200 mg itraconazole BID for 12 months following 1-2 weeks of Amphotericin B | |
WHEN BLASTOMYCOSIS PATIENTS WERE TREATED WITH ITRACONAZOLE3:
90%
ACHIEVED TREATMENT SUCCESS
95%
ACHIEVED TREATMENT SUCCESS WHEN TREATED AT THERAPEUTIC LEVELS FOR AT LEAST 2 MONTHS
6.2 MONTHS
MEDIAN DURATION TREATMENT TIME FOR PATIENTS WHO WERE CONSIDERED A SUCCESS
ITRACONAZOLE REMAINS THE DRUG OF CHOICE FOR TREATING HISTOPLASMOSIS AND BLASTOMYCOSIS AFTER NEARLY THREE DECADES1,2
ASPERGILLOSIS
AREA ENDEMIC FOR INFECTION:

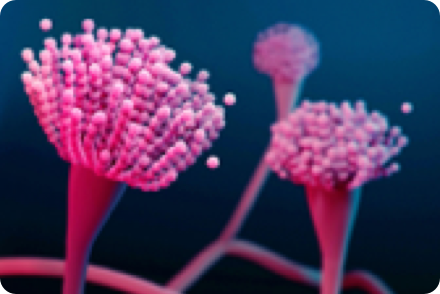
ASPERGILLOSIS
Aspergillosis is a non-endemic, opportunistic mold infection that is common across the US4.
The causative fungi, Aspergillus, is a common mold found both indoors and outdoors. While not contained to a certain geographic area, Aspergillus continues to be a cause of life-threatening infections in patients.
IDSA AND ATS RECOMMEND ITRACONAZOLE FOR ASPERGILLOSIS1,2
IDSA and ATS guidelines describe using antifungals, including itraconazole, for treating cases of aspergillosis.
ASPERGILLOSIS
(Please scroll horizontally to view full table)
| US CLINICAL GUIDELINES FOR THE TREATMENT OF ASPERGILLOSIS SYNDROMES: | ||
|---|---|---|
| Disease Manifestation | Treatment Recommendation (ATS) | Treatment Recommendation (IDSA) |
| Chronic necrotizing (‘‘semi-invasive’’) pulmonary aspergillosis | 200 mg itraconazole TID for 3 days and then OD or BID for at least 12 months, but some prefer 18-24 months in view of the risk for relapse (Strength of Recommendation A, Quality of Evidence II) | Itraconazole alternative therapy option |
| Allergic bronchopulmonary aspergillosis | Itraconazole used as a steroid-sparing agent | Itraconazole as primary therapy |
| Invasive aspergillosis | Primary Therapy: IV voriconazole until improvement followed by oral voriconazole OR oral itraconazole until resolution or stabilization of all clinical and radiographic manifestations | Itraconazole alternative therapy option |
ITRACONAZOLE FOR THE TREATMENT OF ASPERGILLOSIS
WHEN CHRONIC ASPERGILLOSIS PATIENTS WERE TREATED WITH ORAL ITRACONAZOLE5:
63%
global improvement in aspergilloma5
71%
improvement or stabilization of clinical and mycologic features with long term therapy in CPA7
93%
improvement in clinical and radiological features for Chronic Necrotizing Pulmonary Aspergillosis8
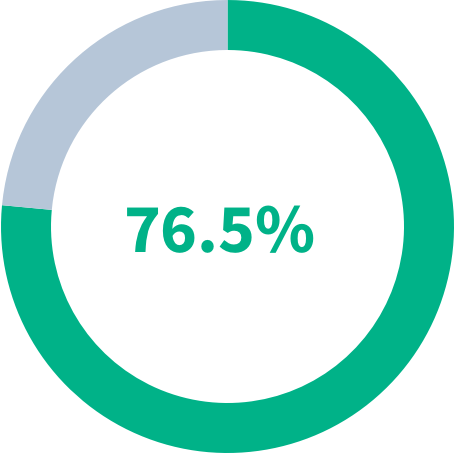
AFTER 6 MONTHS OF ORAL ITRACONAZOLE TREATMENT IN CCPA PATIENTS6:
77%
had improved overall response
76%
had a stable to improved clinical responses
76%
had a stable to improved radiological response
OVERALL RESPONSE IN PATIENTS AFTER 6 MONTHS OF ITRACONAZOLE TREATMENT
- Improved
- Not Improved
FUNGAL INFECTIONS POSE A SIGNIFICANT THREAT FOR SOME PATIENTS9
IMMUNOCOMPETENT AND IMMUNOCOMPROMISED PATIENTS MAY BE AT RISK9,13:
- Organ transplant
- Chemotherapy
- Hematopoietic stem cell transplant
- High-dose corticosteroids
- HIV/AIDS
- Prolonged antibiotic therapy
- Hematologic malignancies
- TPN = total parenteral nutrition (IV nutrition)
- Surgery
- ESRD = end-stage renal disease (kidney failure)
- ICU stay
- Mechanical ventilation
CHALLENGES AND LIMITATIONS WITH CONVENTIONAL ITRACONAZOLE
Sporanox® 100 mg Capsules14
- Bioavailability55%
- Food effect on bioavailability40% decrease when taken without food
- Effect of PPIs on bioavailability68% decrease when taken with omeprazole17
- Inter-Patient variability14,16High
- Absorption variability16,18
- Vary by as much as 15-fold depending on pH of gastric environment
- Requires stomach-acid to release drug, but precipitates in higher pH of intestines
- Patient adherence22-
Sporanox® Oral Solution
- Bioavailability72%23
- Food effect on bioavailability25% decrease when taken with food17
- Effect of PPIs on bioavailability-
- Inter-Patient variability14,16High
- Absorption variability16,18-
- Patient adherence22Palatability issues
TOLSURA ADDRESSES CHALLENGES AND LIMITATIONS WITH CONVENTIONAL ITRACONAZOLE
CONVENTIONAL ITRACONAZOLE
- No advanced delivery technology14,15
- Poor bioavailability14
- Affected by food14,15
- Affected by co-administration with PPI14
- Requires low pH (e.g., acidic stomach) to release and absorb drug16,17
- Precipitates in higher pH of intestines16,17
- High absorption variability16,18
- High patient variability18
TOLSURA
- Unique SUperBioAvailable® delivery technology19
- ~ 2x greater relative bioavailability20
- Maintained similar therapeutic Ctrough levels in fed and fasted states21
- Absorption not reduced by co-administration with PPI17,21
- Does not rely on an acidic stomach for dissolution due to SUBA® technology17,20
- Improved solubility in higher pH of intestines17,20
- Improved absorption due to improved solubility17,20
- Maintains therapeutic levels19

THE SCIENCE OF SUBA®
 WATCH MECHANISM OF ACTION VIDEO
WATCH MECHANISM OF ACTION VIDEO Indications and Important
Safety Information
BOXED WARNING
WARNING: CONGESTIVE HEART FAILURE and DRUG INTERACTIONS
- » CONGESTIVE HEART FAILURE
TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur or worsen during administration of TOLSURA, reassess the benefit and risk of continuing treatment.
- » DRUG INTERACTIONS
- Co-administration of certain drugs that are metabolized by human CYP3A4 enzymes are contraindicated with TOLSURA because plasma concentrations of such drugs are increased, which may also increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs.
- Co-administration with colchicine, fesoterodine and solifenacin is contraindicated in subjects with varying degrees of renal or hepatic impairment, and
- Co-administration with eligustat is contraindicated in subjects taking strong or moderate CYP2D6 inhibitors.
- Increased plasma concentrations of some of these drugs caused by co-administration with TOLSURA can lead to QT prolongation and/or ventricular tachyarrhythmias, including occurrences of torsades de pointes, a potentially fatal arrhythmia.
Indications and Usage
TOLSURA is an azole antifungal indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised adult patients:
- Blastomycosis, pulmonary and extrapulmonary
- Histoplasmosis, including chronic cavitary pulmonary disease and disseminated, non-meningeal histoplasmosis, and
- Aspergillosis, pulmonary and extrapulmonary, in patients who are intolerant of or who are refractory to amphotericin B therapy
Limitations of Use:
TOLSURA is not indicated for the treatment of onychomycosis.
TOLSURA is NOT interchangeable or substitutable with other itraconazole products.
Contraindications
Co-administration with certain drugs that either affect metabolism of itraconazole or whose metabolism is affected by itraconazole Hypersensitivity to itraconazole
Warnings and Precautions
- Hepatotoxicity: Serious hepatotoxicity, including liver failure and death, were reported with the use of itraconazole. Discontinue treatment if signs of liver dysfunction occur
- Cardiac Dysrhythmias: Life-threatening cardiac dysrhythmias and/or sudden death have occurred in patients using certain drugs that are metabolized by human CYP450 enzymes concomitantly with oral itraconazole and/or other CYP3A4 inhibitors.
- Peripheral Neuropathy: This has been reported in patients on long-term therapy with itraconazole. Monitor and promptly evaluate neurologic symptoms
- Hearing loss: Reversible or permanent hearing loss has been reported in patients. Discontinue treatment if hearing loss occurs
Adverse Reactions
Most common adverse reactions (incidence ≥ 1%) are nausea, rash, vomiting, edema, headache, diarrhea, fatigue, fever, pruritus, hypertension, abnormal hepatic function, abdominal pain, dizziness, hypokalemia, anorexia, malaise, decreased libido, somnolence, albuminuria, and impotence.
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For more information, please see full Prescribing Information and Patient Information Leaflet.
References:
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(12)1801-1812.
- Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7)807-825.
- Dismukes WE, et al. Am J Med. 1992;93(5):489-497.
- Patterson TF, et al. Clin Infect Dis. 2016;63(4):433-442.
- Tsubura E, et al. Kekkaku. 1997;72:557-64.
- Agarwal R, et al. Mycoses. 2013;56(5):559-570.
- Denning D, et al. Clin Infect Dis 2003;37(S3): S265–80.
- Dupont B, J Am Acad Dermatol 1990;23:607–14.
- Badiee P, Hashemizadeh Z. Opportunistic invasive fungal infections: diagnosis & clinical management. Indian J Med Res. 2014;132(2):195-204.
- Centers for Disease Control and Prevention. Symptoms of histoplasmosis. https://www.cdc.gov/fungal/diseases/histoplasmosis/symptoms.html. Accessed September 26, 2020.
- Centers for Disease Control and Prevention. Symptoms of blastomycosis. https://www.cdc.gov/fungal/diseases/blastomycosis/symptoms.html. Accessed September 26, 2020.
- Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34(1):7-14.
- Cornely OA. Aspergillus to Zygomycetes: causes, risk factors, prevention, and treatment of invasive fungal infections. Infection. 2008;36(4)296-313.
- Sporanox® Product Information. Janssen Pharmaceuticals, Inc. Titusville, NJ. May 2018.
- Sporanox® oral solution Product Information. Janssen Pharmaceuticals, Inc. Titusville, NJ. Apr 2019.
- Prentice AG, Glasmacher A. J Antimicrob Chemother. 2005;56(Suppl 1):i17-i22.
- Abuhelwa AY, et al. Eur J Drug Metab Pharmacokinet. 2019;44(2):201-215.
- Poirier JM, et al. Therapie. 1996;51(2):163-167.
- TOLSURA® Product Information. Mayne Pharma. Greenville, NC. 12/2018.
- Abuhelwa AY, et al. J Pharmacokinet Pharmacodyn. 2018;45(2):181-197.
- Lindsay J, et al. Antimicrob Agents Chemother. 2018;62(12)e01723-18.
- Gould IM, van der Meer JWM. Antibiotic Policies: Theory and Practice. New York: Kluwer Academic/Plenum, 2005.
- Thompson GR, et al. Antimicrob Agents Chemother. 2020;64(8):e00400-20.
Indications and Important
Safety Information
BOXED WARNING
WARNING: CONGESTIVE HEART FAILURE and DRUG INTERACTIONS
- » CONGESTIVE HEART FAILURE
TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur or worsen during administration of TOLSURA, reassess the benefit and risk of continuing treatment.
- » DRUG INTERACTIONS
- Co-administration of certain drugs that are metabolized by human CYP3A4 enzymes are contraindicated with TOLSURA because plasma concentrations of such drugs are increased, which may also increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs.
- Co-administration with colchicine, fesoterodine and solifenacin is contraindicated in subjects with varying degrees of renal or hepatic impairment, and
- Co-administration with eligustat is contraindicated in subjects taking strong or moderate CYP2D6 inhibitors.
- Increased plasma concentrations of some of these drugs caused by co-administration with TOLSURA can lead to QT prolongation and/or ventricular tachyarrhythmias, including occurrences of torsades de pointes, a potentially fatal arrhythmia.
Indications and Usage
TOLSURA is an azole antifungal indicated for the treatment of the following fungal infections in immunocompromised and non-immunocompromised adult patients:
- Blastomycosis, pulmonary and extrapulmonary
- Histoplasmosis, including chronic cavitary pulmonary disease and disseminated, non-meningeal histoplasmosis, and
- Aspergillosis, pulmonary and extrapulmonary, in patients who are intolerant of or who are refractory to amphotericin B therapy
Limitations of Use:
TOLSURA is not indicated for the treatment of onychomycosis.
TOLSURA is NOT interchangeable or substitutable with other itraconazole products.
Contraindications
Co-administration with certain drugs that either affect metabolism of itraconazole or whose metabolism is affected by itraconazole Hypersensitivity to itraconazole
Warnings and Precautions
- Hepatotoxicity: Serious hepatotoxicity, including liver failure and death, were reported with the use of itraconazole. Discontinue treatment if signs of liver dysfunction occur
- Cardiac Dysrhythmias: Life-threatening cardiac dysrhythmias and/or sudden death have occurred in patients using certain drugs that are metabolized by human CYP450 enzymes concomitantly with oral itraconazole and/or other CYP3A4 inhibitors.
- Peripheral Neuropathy: This has been reported in patients on long-term therapy with itraconazole. Monitor and promptly evaluate neurologic symptoms
- Hearing loss: Reversible or permanent hearing loss has been reported in patients. Discontinue treatment if hearing loss occurs
Adverse Reactions
Most common adverse reactions (incidence ≥ 1%) are nausea, rash, vomiting, edema, headache, diarrhea, fatigue, fever, pruritus, hypertension, abnormal hepatic function, abdominal pain, dizziness, hypokalemia, anorexia, malaise, decreased libido, somnolence, albuminuria, and impotence.
To report SUSPECTED ADVERSE REACTIONS, contact Mayne Pharma at 1-844-825-8500 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For more information, please see full Prescribing Information and Patient Information Leaflet.
